The “Polimery Police” project implemented by Grupa Azoty Polyolefins S.A. covers two main technological processes:
The raw material and product chain can be divided into three streams:
PROPANE
LPG (Liquefied Petroleum Gas) is a mixture of propane and butane molecules containing traces of other chemical compounds. It is a naturally occurring by-product associated with natural gas and crude oil production. LPG originates from two sources:
Before natural gas and oil are transported or used, gases that form LPG are separated using various chemical processes: crude oil distillation, gasoline reforming, cracking, gaseous or liquid hydrocarbon pyrolysis,
In 2017, the world LPG production amounted to approximately 160 million tons, of which approximately 60 million tons of this raw material were used in chemical processes, mainly in steam cracking units and on-purpose propylene production technologies. The European market supplies approx. 10 million tons of LPG.
LPG belongs to the group of liquid hydrocarbons which at room temperature and normal pressure are in the form of a gas. A characteristic feature of liquefied gas covers its easy ability to transform from gaseous to liquid phase under pressure (called saturation pressure) of approximately 2 to 4 bar at room temperature.
Taking into account the project assumptions, in the case of the investment project carried out by Grupa Azoty Polyolefins S.A., LPG with a minimum propane content of 95% by weight will be stored on the premises of the Handling and Storage Terminal in liquid state in above-ground tanks under atmospheric pressure at a temperature of approximately -42 degrees Celsius.
Liquid propane from the Handling and Storage Terminal will be sent to the PDH Plant for dehydrogenation to propylene.
Curiosity: LPG in the gaseous phase is colorless and odorless. Although used in numerous technological processes, it is most often associated by society as a source of power supply for heating devices and gas cookers, as well as motor fuel - autogas. For safety reasons, for this type of applications, LPG is odorized with an organic chemical compound - ethanethiol (ethyl mercaptan), which provides an extremely strong, unpleasant odor.
PROPYLENE
After ethylene, propylene is the second most important unsaturated hydrocarbon and is a raw material for the petrochemical industry, with world production of around 105 million tons in 2017. The main source of propylene supply are steam crackers, which account for approximately 48% of world production of this raw material. In catalytic cracking units, approximately 29% of the propylene sold on the market is produced. The remaining 23% originates from on-purpose units. Currently, although approximately 80% of propylene production is produced in steam and catalytic cracking processes, this substance is a by-product formed by means of the above mentioned technologies. Considering the global increase in propylene demand in various industry branches, on-purpose technologies aimed at generation of the highest yield of this olefin are becoming increasingly important.
PROPYLENE PRODUCTION TECHNOLOGY
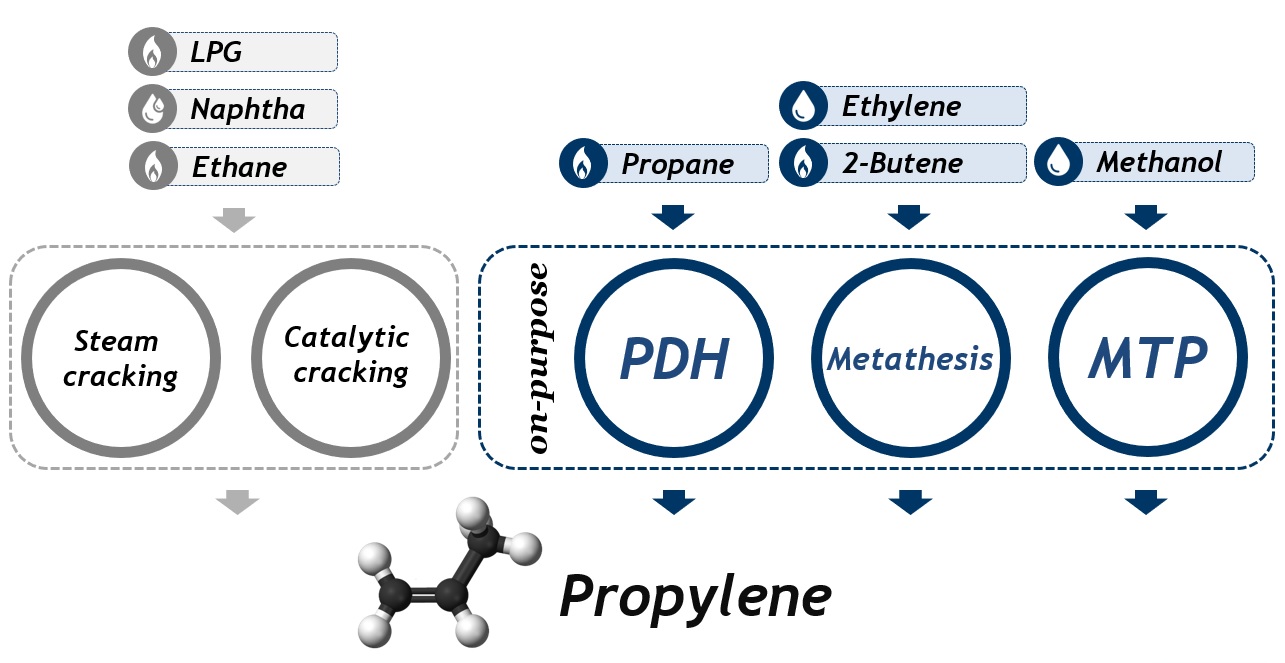
The most important on-purpose methods for propylene production are as follows:
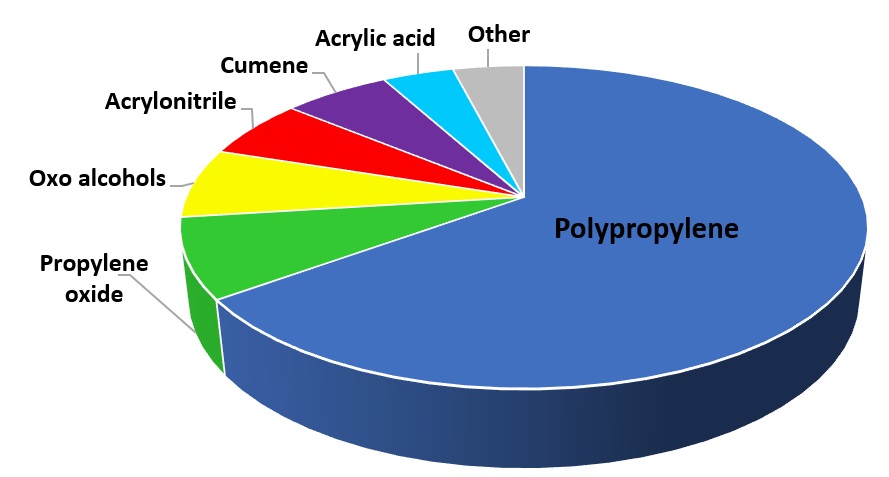
Propylene is used for the production of a wide range of other chemical products, including, i.a.:
WORLD CONSUMPTION OF PROPYLENE IN 2017 ACCORDING TO ITS INTENDED PURPOSE
SELECTION OF PDH TECHNOLOGY UNDER UOP LICENSE
Among the considered technologies, which result in obtaining propylene, the PDH method under the conditions applicable for the Polimery Police project is characterized by the best process and economic indicators, which makes this technology more advantageous when compared to cracking processes and other on-purpose technologies.
Among the suppliers of propane dehydrogenation technologies, one can distinguish, i.a., UOP (Oleflex® technology), McDermott (CATOFIN® technology) and ThyssenKrupp (STAR process® technology). Of all propane dehydrogenation technologies, Oleflex® method is the world's leading.
In the case of Grupa Azoty Zakłady Chemiczne "Police" S.A. and the established special purpose vehicle named Grupa Azoty Polyolefins S.A., the technology based on UOP license was chosen as the best solution ensuring economic effectiveness, limitation of negative impact on the environment and process safety. The selection was made taking into account the information provided by the licensors (technical proposals) and the recommendations of technical advisors.
Oleflex® technology under the UOP license is designed for the production of polymer grade propylene. In comparison to other PDH methods, this technology ensures:
1. Low operating costs due to low raw material consumption and low energy consumption;
2. Low investment costs due to the use of highly active and stable catalyst
Curiosity: Platinum (Pt) is an extremely rare noble metal. Its amount in the catalyst for propane dehydrogenation is over 400 kg.
3. High reliability of the unit due to modern design solutions and the possibility of catalyst regeneration without the need of stopping the propylene production process:
4. Low environmental impact:
The propylene production process using Oleflex® technology consists of four main sections:
The C3- (propane and lighter hydrocarbons) raw material stream, cleaned of unwanted impurities and dried, additionally separated from heavier fractions of C4+ (butane and heavier hydrocarbons), is send to the reactor section, where the endothermic process of propane dehydrogenation to propylene takes place. This section consists of a system of reactors operating continuously and filled with a moving platinum-based catalyst bed. The catalytic reaction of propane dehydrogenation is accompanied by the process of coke deposition on the catalyst surface and its deactivation.
Used (deactivated) catalyst from the reaction section is transported to the regeneration section, where a number of processes related to the restoration of its activity take place in a continuous mode, i.e:
The regenerated catalyst is returned to the reaction section.
After leaving the reactor system, the post-reaction mixture is compressed, cleaned, dried and sent to a cryogenic separation system, where two streams are generated: liquid and gaseous. Liquid stream rich in produced propylene and unreacted propane is sent to the fractionation section. The gaseous stream containing significant amounts of hydrogen, after being used in other unit stations, is subjected to pressure swing adsorption process, which results in high-purity hydrogen.
The fractionation section is designed to clean the
propylene-containing stream by means of a system of two cold boxes: a deethanizer and a P-P splitter. The purpose of the deethanizer is to
separate the volatile component stream from the product stream, while
the purpose of the second box is to separate unreacted propane and other
heavier components from propylene. The propylene content in the
obtained product is 99.6% by weight.
POLYPROPYLENE CHARACTERISTICS
Worldwide, there is a growing demand for plastics, which increasingly substitute other materials.
STRUCTURE FOR PLASTICS DEMAND IN EUROPE - 2016
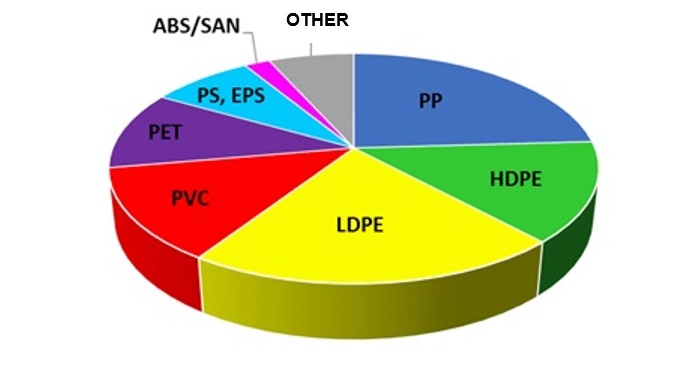
Since the 1960s, the production and consumption of plastics has accelerated dramatically, mainly due to their massive implementation in numerous economy sectors. The consumption of plastics is growing very rapidly, and reached the level of approx. 330 million tons in 2016. World demand for plastics has more than tripled since 1989.
GLOBAL PRODUCTION OF PLASTICS
Polypropylene (PP) is one of the most common polymers in the world and the most common propylene derivative, created in the process of this raw material polymerization. In addition, with a global market estimated at 66.5 million tons in 2016, polypropylene is the second most popular plastic after polyethylene. Polypropylene is used in many industry branches and is one of the most important raw materials used, i.a., in packaging, textile, electronics, household appliances, construction and automotive industries.
There are three main types of polypropylene, which vary in terms of physical and chemical properties of the product:
The possibility of polypropylene modification with the use of, i.a., nanotechnologies, allows for the development of innovative types of plastics with various properties. Therefore, a wide range of polypropylene types is offered on the market.
The properties of the product are primarily influenced by: molecular weight, polydispersity degree and spatial structure. All PP types are characterized by high chemical resistance due to the non-polar nature. They do not exhibit sensitivity to strong, but non-oxidizing acids and bases, salt solutions, washing agents, alcohols as well as oils and fats. They have a very low absorbency and permeability of both water and water-based products. Gases, such as CO2 as well as vapors of hydrocarbons and their halogen derivatives also cannot diffuse through PP. At room temperature, they are characterized by high rigidity and mechanical strength. The maximum temperature values of PP use are, in the short-term, up to 140°C, and in the case of a long-term use up to 100°C. Under the influence of oxygen and UV radiation, polypropylene is subjected to partial degradation, and the process accelerates at high temperature. When the fire is removed it burns with slightly glowing flame. In order to stabilize and improve certain properties of polypropylene, various auxiliary agents are added to it.
PRODUCTION PROCESSES AND TECHNOLOGIES USED FOR PP PRODUCTION
Polypropylene is a product produced in the propylene polymerization process. The process takes place in a catalytic system consisting of a transition metal compound anchored on the carrier surface, which repeatedly increases the catalyst activity. Polypropylene producers strive for the most advanced vertical integration possible, which results in frequent placing of polypropylene units in the vicinity of propylene production units.
Curiosity: The discovery by Ziegler and Natt of catalytic systems
containing activated transition metal was a breakthrough in the
development of polyolefins. These scientists received the Nobel Prize
for their work in 1963, thus contributing to the revolutionary
development of catalytic polymerization of olefins.
Technologies for polypropylene production can be divided into three groups:
The majority of the currently used technologies is of a modular nature, and typical configuration consists of four basic steps:
Two technologies dominate among PP producers: UNIPOL PP by Grace and
Spheripol by LyondellBasell. Based on these technologies, over 55% of
global polypropylene production is effected. Popularity of these methods
results from the limited number of operations and unit processes, and
the use of modern catalysts ensures perfect properties of the produced
PP types.
SELECTION OF PP UNIPOL TECHNOLOGY UNDER GRACE LICENSE
The UNIPOL technology by Grace company is based on gaseous phase polymerization and ensures production of a wide range of highest grade polypropylenes in an easy and economical manner. The versatility of the process is clearly demonstrated by the ease of its control, low investment and operating costs and the possibility of producing a full range of standard polypropylenes, as well as numerous unique, special products. In this process, homopolymers, random copolymers and impact copolymers can be produced.
Two fluid-bed reactors in series are used in the UNIPOL process by Grace company. Both reactors operate in the environment of gaseous monomer and/or comonomer. A catalytic system (catalyst, co-catalyst and donor), propylene (monomer), ethylene (comonomer) and hydrogen for the control of molecular weight are fed to the reactor in a continuous manner.
During the production of random copolymers or terpolymers, minor amounts of comonomers, such as ethylene are fed into the process. In addition, during the production of block copolymers (the so-called impact or heterophase copolymers), the polymer from the first reactor is sent to the second reactor where a layer of elastomer is formed on the previously prepared polypropylene matrix.
The polymer powder from the reactor (first or second, depending on the produced PP type) is sent to the separator, where the remains of the catalyst are neutralized, and the monomer is removed, recovered and returned to the reaction system. In the final stage of the process, the final product is transported to the extrusion set, where it is mixed with additives and extruded in the form of granules.
